Emerging Approach to Cancer Therapy - Targeted Protein Degraders
By Alexander An on September 11th, 2023
Chemotherapy, radiation, and surgery have been the mainstays of cancer treatment. Patients have endured long-term toxicities as a result of chemotherapy and radiotherapy’s nonspecific targeting. To solve this problem, small molecule inhibitors like lapatinib and ibrutinib were created, which target proteins implicated in the pathogenesis of cancer while sparing healthy cells. One drawback of small molecule inhibitors is that the drug requires a binding pocket in the target protein. As a result, small molecule inhibitors fail to target 85% of the proteome, which includes proteins like scaffolding proteins and transcription factors. Another disadvantage is that a large concentration of it is needed to have therapeutic effects, yet doing so can increase toxicities and adverse effects. The last limitation is that prolonged use of those small molecule inhibitors may select for mutation in the target protein that results in drug resistance.
Figure on targeting proteins in cancer pathogenesis with protein degraders from Protein degraders enter the clinic — a new approach to cancer therapy
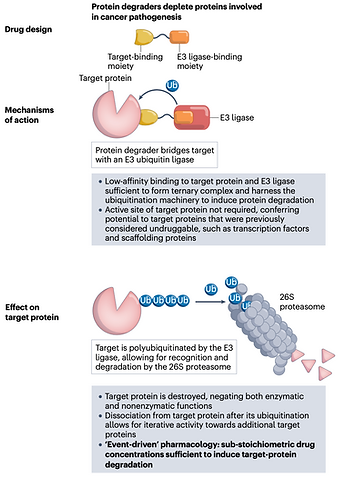
By destroying the target protein rather than inhibiting it, new drug classes known as Proteolysis Targeting Chimera (PROTAC) protein degraders can resolve those problems. Compared to small molecule inhibitors, PROTAC can target undruggable targets such as STAT3/KRAS, resistance mutation, single protein isoforms, proteins with scaffolding function, and protein aggregates. This is accomplished by using the ubiquitin-proteasome system to get rid of disease-causing proteins. PROTAC, also known as heterobifunctional protein degraders, has two ligands connected by a linker, one of which attracts the desired protein and the other of which attracts and binds an E3 ubiquitin ligase. Both binding by PROTAC causes the target protein to be ubiquitylated and degraded via the ubiquitin-proteasome system. Protein degraders can enhance the breakdown of several target molecules in a sub-stoichiometric way because the proteasomal degradation that follows the initial degradation is kinetically irreversible. As a result, classical pharmacology’s occupancy-driven paradigm no longer fits protein degraders, which are better defined as having an event-driven pharmacology.
Bruton’s tyrosine kinase (BTK), which is a crucial player in the signaling of B cell proliferation and survival, is one target under investigation for protein degradation. With various medicines, including BTK inhibitors like ibrutinib, approved for the treatment of B malignancies, BTK has been established as a proven clinical target for hematological malignancies. The emergence of resistance mutations in BTK, however, limits the effectiveness of such drugs. Since the first PROTAC entered clinical testing in 2019, there have been around 18 targeted protein degraders in trials for various cancer indications. Four BTK protein degraders are now being tested in clinical trials to address this problem. One notable example is NX-2127, a BTK protein degrader created by Nurix.
Nurix is a biotech company that specializes in treating various diseases by targeting protein degradation. One of the company’s leading assets is NX-2127, an oral small molecule combining BTK and IKZF degrader, for treating B cell malignancies. NX-2127 has been demonstrated to induce the degradation of multiple BTK inhibitor-resistant mutants including C481S variant.
With a data cut-off date of September 21, 2022, the most recent results from the phase 1 study of the BTK degrader NX-2127 reported data from 36 patients with relapsed and/or refractory B cell malignancies (of whom 23 had chronic lymphocytic leukemia (CLL)) who were treated with oral doses of 100 mg, 200 mg, or 300 mg of NX-2127 once daily. A BTK inhibitor was previously administered to 86% of patients (100% in the subgroup with CLL), and 35% of patients exhibited a BTK inhibitor resistance mutation (48% in the subgroup with CLL). The median number of prior lines of therapy was four in the general population (five in the subgroup with CLL). Fatigue (53%), neutropenia (39%), contusion (28%), thrombocytopenia (25%), hypertension (25%), and anemia (22%) were the treatment-emergent adverse events observed in patients. The objective response rate was 33% (95% CI 12–62%) in the 15 patients with CLL after a median follow-up duration of 5.6 months (range 0.3–15.7). While the rest of the patients remained on NX-2127 treatment. Regardless of BTK mutation status, NX-2127 induced BTK degradation and had clinical effects.
Check out my personal blog page as well here: https://medium.com/@alexanderan